T-STENT TECHNOLOGY
A modular and patented innovation to restore natural venous flow and sustainably improve patient quality of life.
AN INNOVATION BORN FROM CLINICAL PRACTICE
The T-Stent is the result of over thirty years of surgical experience. Faced with the limitations of existing devices – migration, over-expansion, incomplete apposition – the ID NEST teams rethought the stent from its primary function: to conform to venous physiology, without constraining it. This biomimetic approach translates into a unique design that is simple to implant and durable over time.
MODULAR AND ADAPTIVE ARCHITECTURE
Designed to adapt to bifurcations, compressions, and curvatures, the T-Stent combines flexibility, stability, and optimal anchoring. Its independent module architecture allows for precise adaptation to every venous morphology. The result: restored blood flow, better apposition, and a reduction in the risk of migration.
Each module is designed to work synergistically, offering uniform expansion and controlled resistance to external compression forces.
Result: restored blood flow, perfect apposition, elimination of the risk of migration.
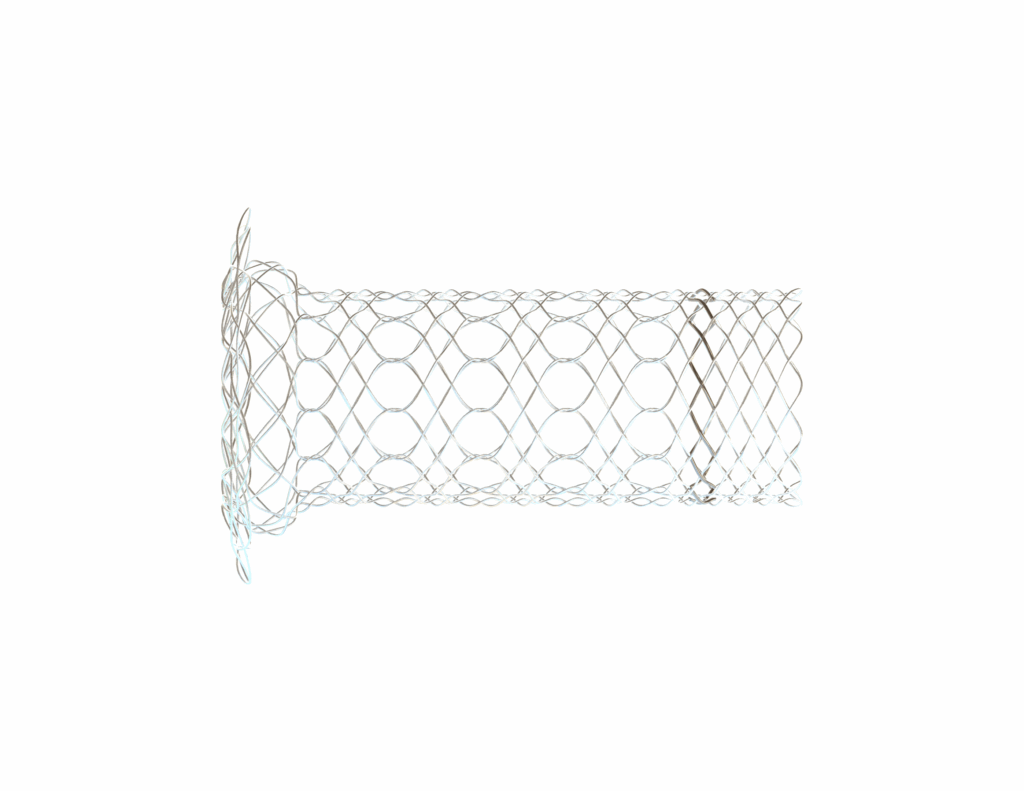
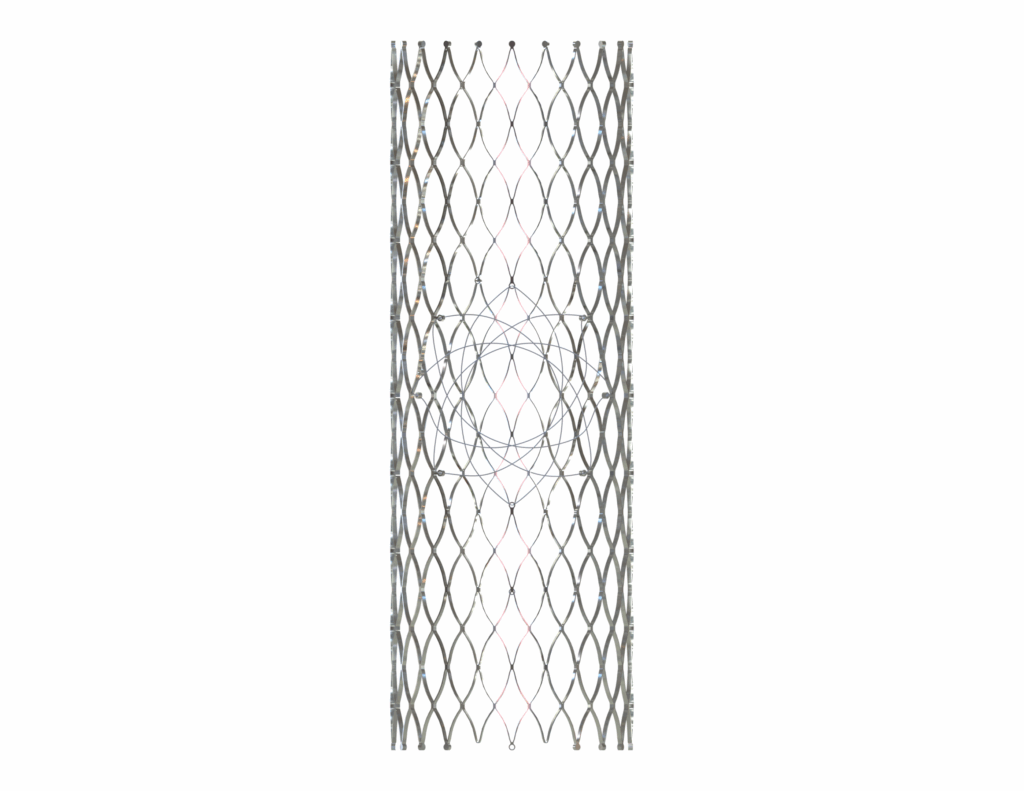
The BRANCH STENT (Pathological)
Common to May-Thurner & Nutcracker syndromes
This cylindrical braided stent is universal for all venous confluences: iliac veins, left renal vein, and other venous branches.
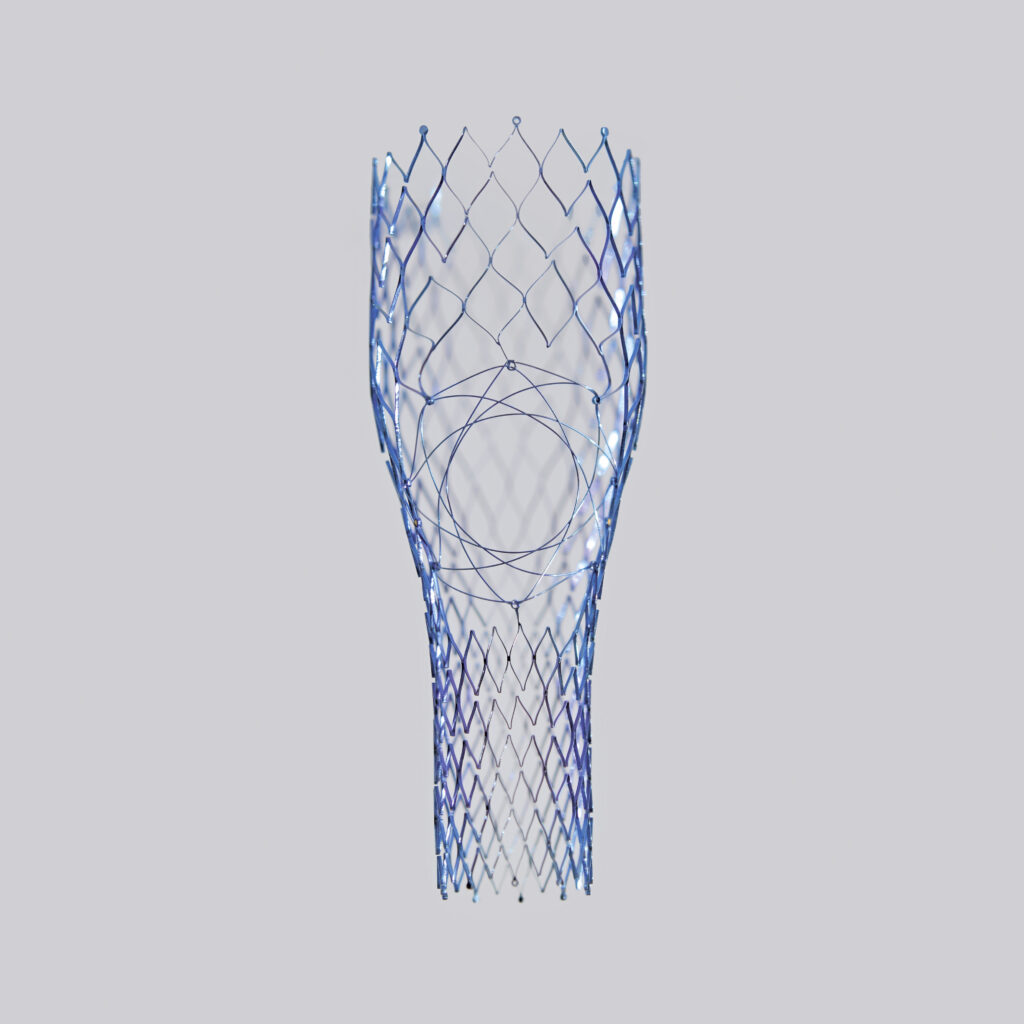
The MOTHER STENT (Conical)
Specific to May-Thurner (Iliac Vein)
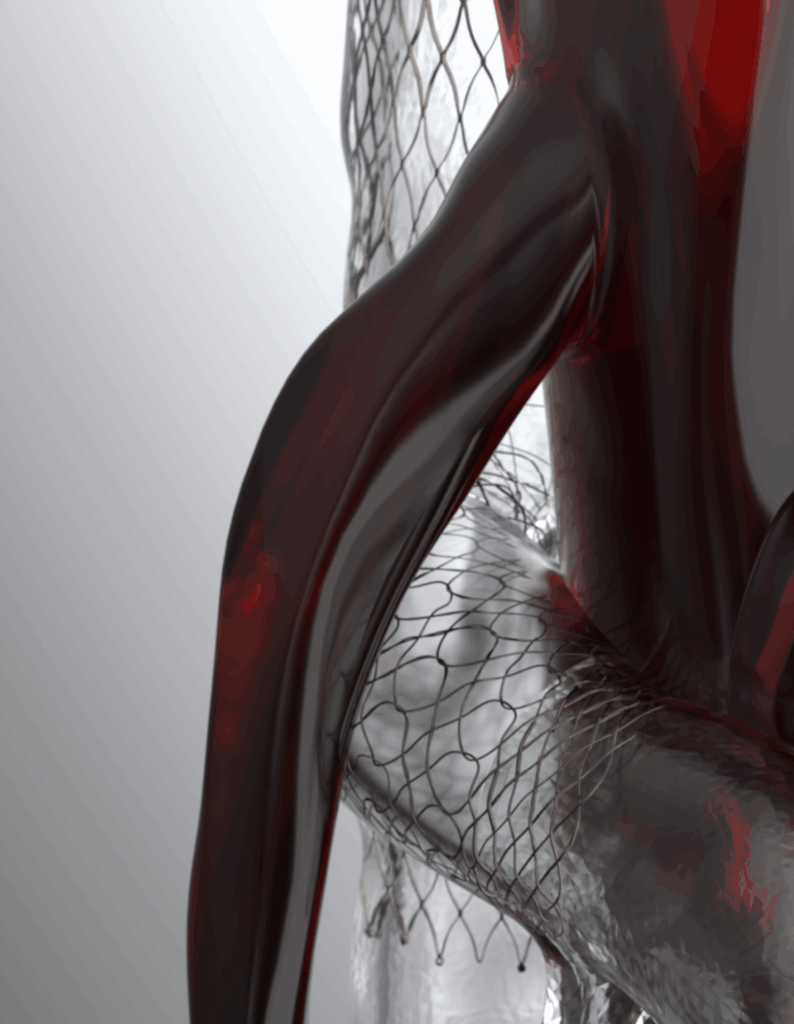
Specific to the iliocaval confluence (May-Thurner syndrome, post-thrombotic syndrome), its conical shape conforms to the anatomy of the iliocaval confluence (diameter and angulation). It features an opening with a Nitinol diaphragm that naturally clips between the ridge and the flared end of the branch stent, ensuring stable anchoring and preventing any dislodgement or migration.
MOTHER STENT (Cylindrical)
Specific to Nutcracker (Vena Cava)
Specific to the left reno-caval confluence (Nutcracker syndrome), its tubular geometry is adapted to the anatomy of the inferior vena cava with a Nitinol diaphragm ideally positioned facing the ostium of the left renal vein.
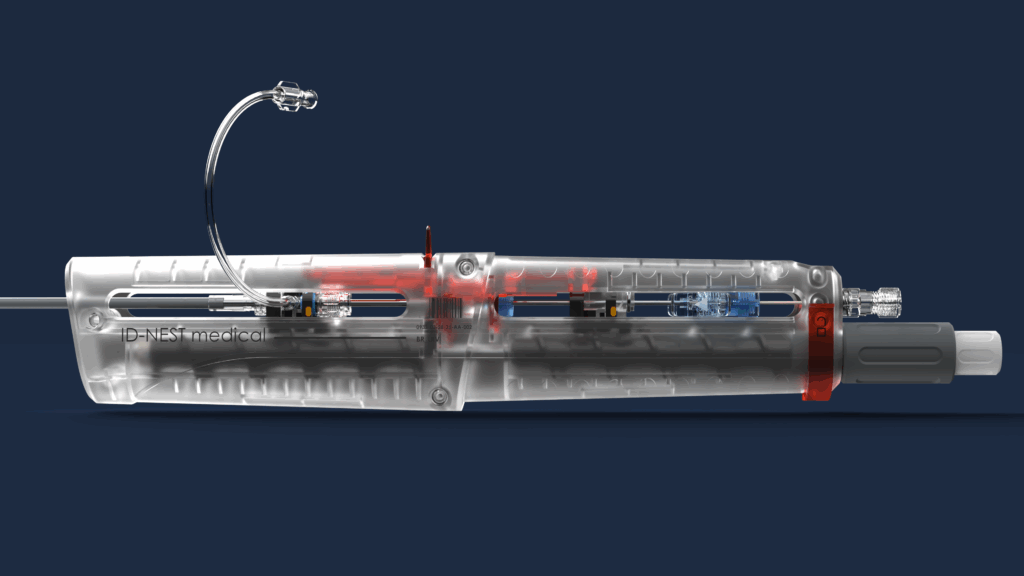
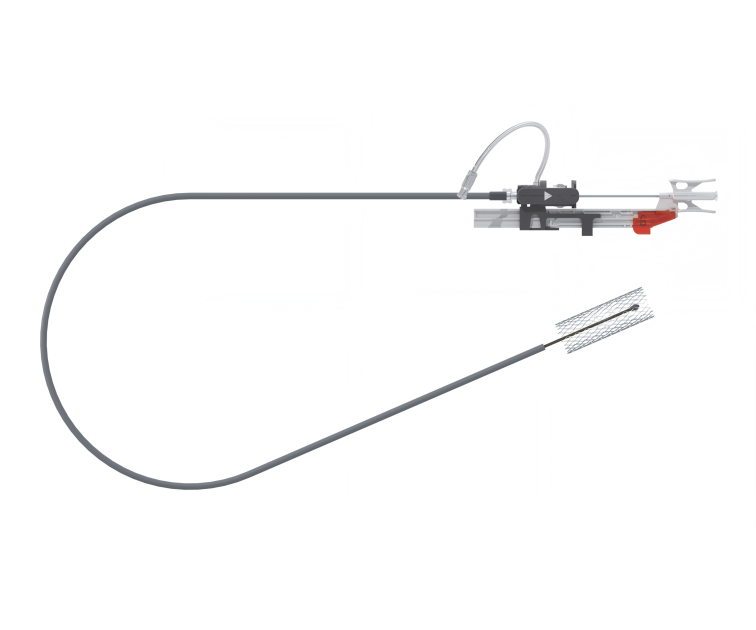
ASSOCIATED DELIVERY SYSTEMS
Innovation goes beyond the implant itself.
To guarantee secure and reproducible deployment, we have developed patented delivery systems. These tools provide to the user with the safety to deploy both elements of the T-stent with precision and simplicity, ensuring a secure and accurate connection. These systems make the T-stent accessible even to less experienced users.
UNIVERSAL DELIVERY SYSTEM
Specific to the mother stent, it allows for its perfect 3-step deployment with perfect alignment of the diaphragm orifice facing the ostium of the venous branch where the branch stent is to be deployed.
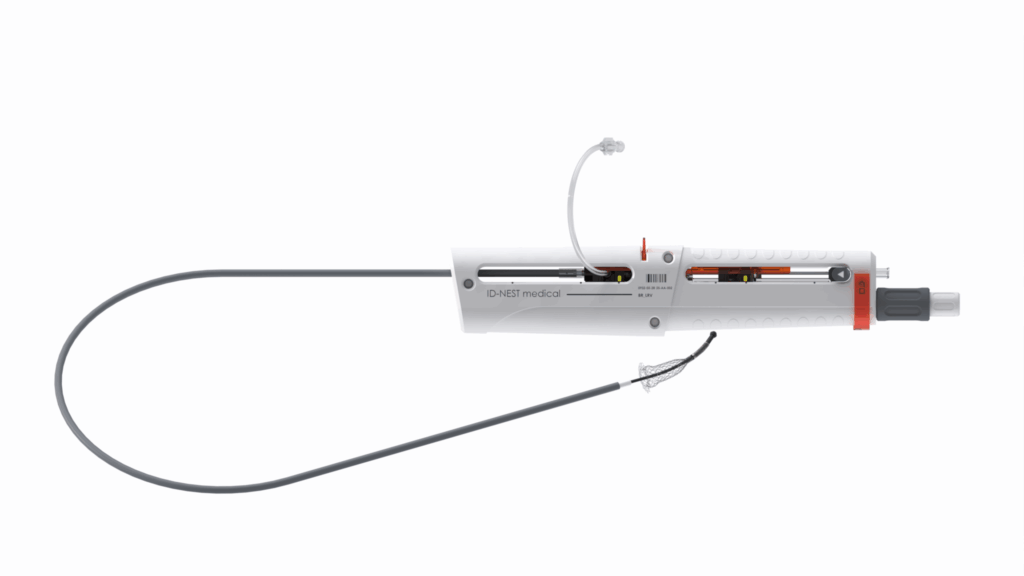
REVERSE DELIVERY SYSTEM
The Branch Stent specification
A major innovation, the reverse delivery system is the only system on the market allowing the sequential 3-step deployment of the branch stent, starting with the deployment of the flower and the collar on either side of the diaphragm before delivering the body, without risk of the delivery system advancing towards the kidney. It is particularly adapted for the treatment of Nutcracker syndrome.
It may be used in future developments of the T-stent (superior vena cava, common femoral vein, and atheromatous and aneurysmal arterial pathologies).
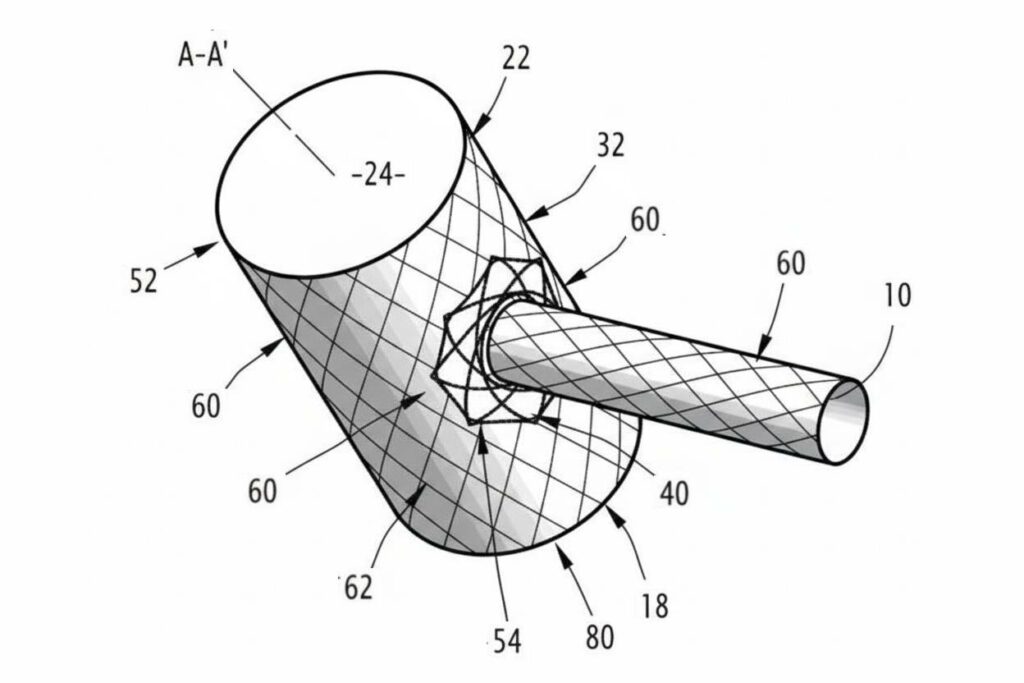
PATENTED TECHNOLOGY
Protected by several international patents, the T-Stent introduces major advances:
- Patented elastic connection allowing better mechanical resilience.
- Reverse delivery system for more precise and secure implantation.
- Self-expanding Nitinol structure guaranteeing optimal flexibility.
These innovations offer the practitioner total control over positioning while ensuring patient safety.
PRECLINICAL TRIALS
Before being deployed in a clinical setting, our T-Stent solution successfully passed a first validation milestone: the Proof of Concept. Protected by international patents, our T-Stent technology is undergoing in-depth testing to guarantee its reliability, safety, and performance under the most demanding conditions.
SAFETY TESTING
Our devices were submitted to strict evaluations to demonstrate their biocompatibility and safety. We ensure that no material presents a risk to the patient, thus respecting the highest regulatory standards.
USABILITY TESTING
Ease and precision of placement were validated in simulated conditions. Usability tests confirmed the optimal maneuverability of our patented delivery systems, guaranteeing a fluid and reproducible procedure for the surgeon.
PERFORMANCE TESTING
System durability was tested on fatigue test benches. These tests simulated millions of respiratory and pulsatile cycles to confirm the resistance of the compliant connection and the absence of long-term migration or fracture.
PURSUING INNOVATION FOR PATIENTS
ID NEST Medical collaborates with reference clinical and scientific centers to continuously validate and improve T-Stent technology. Our ambition: to guarantee performance, safety, and reliability in the most demanding environments.